A Guide to Extreme Pressure Additives in Lubricants: How They Protect Metal Surfaces
Extreme pressure additives are components added to lubricants that help prevent metal-to-metal contact when loads are extremely high. They work chemically with the metal surfaces to form protective films that shield them from scuffing, welding, and wear. EP additives allow the lubricant to withstand short-term pressures above its normal load capacity without failing.
Common examples of extreme pressure additives include:
- Sulphur-based compounds – These include sulphurized fats and oils, organosulfur compounds, and inorganic sulphides. They form metal sulphide films.
- Phosphorus-based compounds – Examples are phosphates, thiophosphates, and metal dithiophosphates. They form metal phosphate films.
- Chlorine-based compounds – These are chlorinated waxes, oils, and paraffins. They form iron chloride films. However, many are now avoided due to toxicity concerns.
- Other film-forming compounds – Examples are fatty acids, amines, graphite, and molybdenum disulphide.
How Do Extreme Pressure Additives Work?
EP additives function through complex chemical reactions that create a protective film on metal surfaces. The additive molecules decompose under the high pressures and temperatures between contacting metal parts and react rapidly with the freshly exposed metal surfaces beneath the lubricant film to form inorganic films chemically bonded to the metal.

These reaction films have a plate-like crystalline structure that provides a smooth glide plane. They shield the underlying metal from direct contact, abrasion, and welding. The films also help absorb shock loads, reducing stress on the lubricant film itself. Some EP additives may also react with themselves to form thicker lubricative films.
The Lifecycle of EP Additives
EP additives undergo a sequence of reactions that help them to bond to the load-carrying surfaces and protect them over the lifetime of the oil. In general, there are five discrete steps:
- Adsorption – Additive molecules initially adsorb onto the metal surfaces through polar bonds. This positions them for further reaction.
- Tribochemical Reaction – Under pressure, additive molecules decompose and react with the metal, forming a new inorganic compound plated onto the surface.
- Crystalline Film Formation – The reaction products deposit as smooth, plate-like crystals bonded to the metal. This crystalline film provides a glide plane.
- Absorption of Shock Loads – The films help absorb pressure spikes, reducing stresses on the base oil film.
- Replenishment – Additives continually replenish the films as they are worn off to maintain protection.
The chemical reactions between the additive and metal depend on the additive type and base metal composition. But in essence, the additives sacrifice themselves to generate inorganic solid films on the metal that provide exceptional wear protection under boundary lubrication conditions.
Sulphur-Based Extreme Pressure Additives
Sulphur-containing compounds are among the most widely used and effective EP additives for lubricants. Common examples include:
- Sulfurized Fats and Oils – These are vegetable or animal fats and oils reacted with elemental sulphur under high temperatures. The sulphur adds sulphide and disulphide groups.
- Organosulfur Compounds – Examples are sulphur-containing olefins, dihydrocarbyl polysulfides, and benzotriazoles. Many contain reactive S-S bonds.
- Inorganic Sulphides – These are inorganic salts like zinc dialkyldithiophosphate and molybdenum disulphide.
The key mechanism of sulphur EP additives relies on tribochemical reactions to form metal sulphide films. Under high pressures between metal parts, the S-S and C-S bonds in the additives break and liberate reactive sulphur. This sulphur then reacts with exposed metal atoms to form metal sulphides following this general reaction:
2Me + S → Me2S
Me = Fe, Cu, Pb, Zn, etc.
These metal sulphides have a layered crystalline structure that provides an excellent lubricating film. Sulphur EP additives can form films on a wide range of alloys. The films have very high adhesion, low shear strength, and replenish rapidly as they are worn off. This provides durable EP protection.
However, the performance of sulphur additives depends heavily on how they are formulated and processed. How the sulphur is incorporated (functionalized, incorporated into the hydrocarbon backbone, or simply blended), the sulphur matrix (interactions between the sulphur and the base oil matrix) can both influence properties like oil solubility. The degree of stability is also a fine-tuned pathway that must thread the needle between decompose under pressure to liberate sulphur but avoid premature reactions during storage. This also impacts the rate of film formation and film removal rates, as the replenishment rate of the films depends on wear rate and additive concentration.
There is also some confusion regarding the differences between “active” and “inactive” sulphur.
Active sulphur EP additives contain reactive sulphur atoms that readily form protective chemical bonds on metal surfaces. For example, pentasulphides have 5 sulphur atoms bonded together that can detach and react with metals under high pressure and temperature. This provides excellent anti-wear capabilities even in harsh environments with heavy loads.
On the other hand, inactive sulphur EP additives like trisulphides have only 3 sulphur atoms bonded together, making them less reactive. Rather than chemical bonding, they rely more on physical adsorption to metal surfaces to form protective films. While less potent than active sulphur additives, inactive versions provide sufficient protection in moderate conditions and are compatible with a wider range of other additives.
The choice between active and inactive sulphur EP additives depends on the specific application and operating parameters. Active sulphur versions excel in extreme pressure and anti-wear performance but can potentially corrode yellow metals like bronze. This can often present an issue in worm drive gearboxes in which the wheel is often composed of a soft yellow-metal. Inactive sulphur additives are a versatile, moderate-performance option suitable for a broad range of industrial uses.
Proper formulation using the right balance of these additives is key to enhancing the wear protection, efficiency and longevity of industrial machinery. Understanding the chemical structure and reactivity of sulphur EP additives allows formulators to tailor blends to meet the demands of specific mechanical systems.
Phosphorus-Based Extreme Pressure Additives
Phosphorus-containing compounds are another large class of EP lubricant additives. Examples include:
- Phosphates – like tricresyl and trialkyl phosphates
- Thiophosphates – such as zinc dialkyldithiophosphate (ZDDP)
- Metal dithiophosphates – e.g. molybdenum dithiophosphates
You may have noticed that ZDDP was already included as an example of a sulphur-based additive. As a molecules containing both sulphur and phosphorous, this compound forms mixed films on metal surfaces.
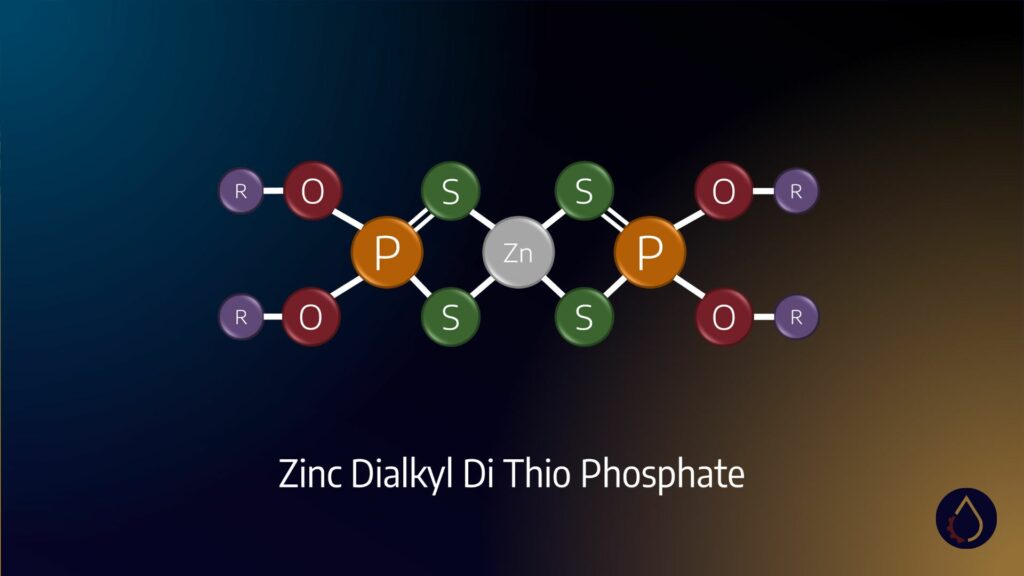
As antiwear and antioxidant additives, phosphorus compounds form protective films through similar tribochemical reactions to sulphur additives. However, they deposit glassy polyphosphate films instead of sulphides.
O=P-S and P-S bonds in the additives decompose under pressure. Phosphorus and oxygen then react with the metal to form a phosphate glass film following this general reaction:
3Me + P + 3O → Me3(PO4)
This smooth polyphosphate film provides good wear resistance and load capacity. However, phosphorus films are generally thicker but less durable than sulphide films. The films may also limit effectiveness at higher temperatures. Performance depends on oil miscibility and film formation rates.
Many industrial EP additives will combine sulphur and phosphorous-based compounds to achieve reactivity and durability.
Chlorinated Paraffins – Once Key EP Additives, Now Avoided
Chlorinated Paraffins (CPs) were once ubiquitous EP additives in industrial lubricants. They contain paraffin waxes or oils with chlorine incorporated into the carbon chain. This gave excellent EP performance when the carbon-chlorine bonds broke down to deposit iron chloride films on metal.
However, most CPs have been phased out of lubricants due to toxicity issues:
- Short-chain CPs bioaccumulate and are potentially carcinogenic and toxic.
- Longer chain CPs are persistent organic pollutants in the environment.
- CPs contaminated many sites through spills and disposal.
While still permitted in some regions, most lubricant companies have switched to safer chemistries to avoid regulatory and liability risks. Iron chloride films also have poorer integrity compared to sulphide or phosphate films.
For these reasons, CP-based EP lubricants have declined significantly. However, this has driven innovation in sulphur, phosphorus, and other additives to replace their performance.
Matching Lubricants to Extreme Pressure Conditions
Not all lubricants need extreme pressure additives. Lower-load applications like hydraulics may function fine on antiwear or rust inhibitors alone. Conversely, heavily loaded gears, bearing, metalworking, or stamping operations require specific EP lubricant chemistries tailored to the metals and loads involved.
Factors in selecting a suitable EP lubricant include:
- Load levels and pressures – Higher loads need higher and more reactive sulphur contents.
- Operating temperatures – High temperatures limit sulphur reaction rates and favour thermally stable phosphorus additives.
- Sliding vs. non-sliding contact – Sliding needs more film formation; non-sliding needs more extreme pressure resistance.
- Metallurgy – Ferrous metals favour sulphur; non-ferrous metals like bronze favour phosphorus additives.
- Environmental exposure – Phosphorus films resist water washout better than sulphides.
- Compatibility – With other additives, seals, paints, filters, etc.
By balancing these factors, lubricant formulators use EP additives like sulphur and phosphorus compounds to achieve the right film formation, wear prevention, and load capacity needed for tough applications. Proper additive selection and treatment allow lubricants to protect vital metal components under extreme pressure.





Responses