Rafe’s View
Polyalkylene Glycols (PAGs) are versatile polymers widely used across a range of industries. They have gained a reputation within the lubricants industry as a high-performance lubricant weighed down by the baggage of incompatibility with other hydrocarbon lubricants, paints and seals. This reputation may be outdated, and a better understanding of the underlying chemistry of PAGs will help operators understand where they can and cannot be used.
What Are PAGs?
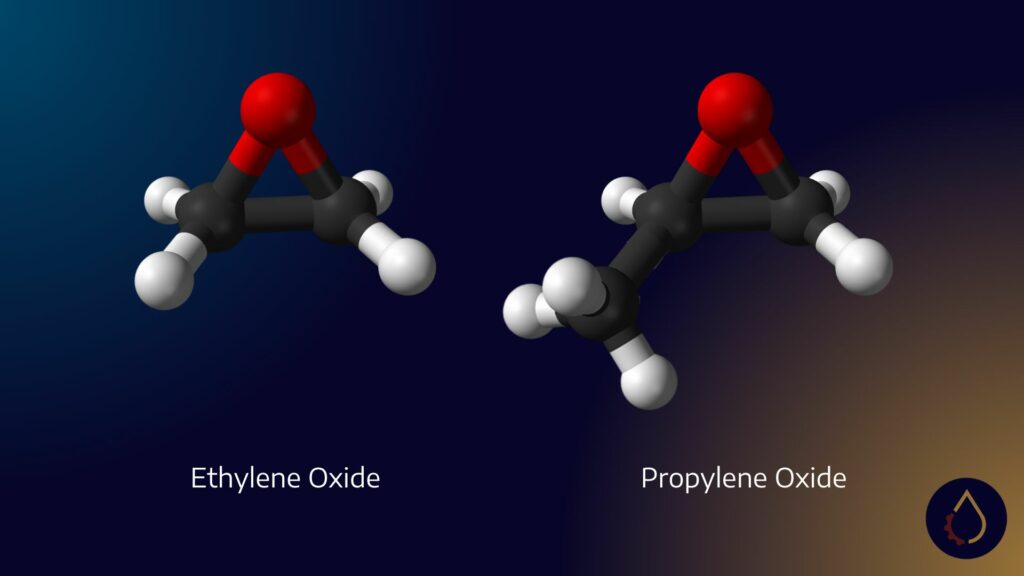
PAGs are a type of polymer, classified as API group five base stocks. They are synthesized using three key oxides: ethylene oxide, propylene oxide, and butylene oxide. These oxides are the building blocks for downstream derivatives known as polymers. In the lubricants industry, these polymers are commonly referred to as PAGs.
How are PAGs Manufactured?
PAGs are synthesized through a process called polymerization, which involves the reaction of the aforementioned oxides to form long-chain molecules. The polymerization process can be tailored to produce PAGs with specific properties, such as viscosity and solubility, by controlling the ratio and sequence of the oxides.
For instance, alternating copolymerization of ethylene oxide and propylene oxide produces a random copolymer PAG, while block copolymerization results in a block copolymer PAG. These two types of PAGs exhibit different properties, such as solubility in water and hydrocarbon fluids, which makes them suitable for various applications.

Once the PAGs are synthesized, they may undergo various post-polymerization treatments to enhance their properties or tailor them for specific applications. These treatments can include filtration to remove impurities, stabilization to improve oxidation resistance, or the addition of additives such as anti-wear agents, corrosion inhibitors, and viscosity modifiers.
What Are PAGs Made From?
There is a well-established infrastructure for producing ethylene oxide and propylene oxide, which serve as precursors for PAGs. These oxides are also utilized in other industries, such as fuel additives, personal care products, paints, coatings, and more. In fact, ethylene oxide and propylene oxide derivatives are found in everyday cleaning products and even in polyurethane foam used for seating.
Butylene oxide is a niche oxide that few chemical companies produce. When combined with PAGs, it results in a new variant called oil-soluble PAGs. Butylene oxide has a unique chemical structure with four carbons, compared to ethylene oxide (two carbons) and propylene oxide (three carbons). This additional carbon imparts diamond-like performance to lubricants, although it may also come with a higher price tag.
What is the Difference Between Water and Oil Soluble PAGs?
Many people in the lubricants industry have heard about oil-soluble PAGs (OSP), a unique type of polyalkylene glycol (PAG) lubricant that is soluble in oil. However, the concept of PAGs not being oil-soluble can be confusing, as PAGs are often thought of as oils themselves.
In the lubricants industry today, there are more than a hundred different chemical families of PAGs, and understanding them all can be quite challenging. To make this easier, PAG manufacturers have segmented PAGs into three primary classes: water-soluble PAGs, water-insoluble PAGs, and oil-soluble PAGs.
- Water-soluble PAGs are manufactured from ethylene oxide and propylene oxide,
- Water-insoluble PAGs are made from propylene oxide alone. These water-insoluble PAGs are neither water-soluble nor oil-soluble.
- Oil-soluble PAGs are truly oil-soluble but not water-soluble.
What Other PAG Variants Can be Manufactured?
When discussing the hundreds of different types of PAGs, it is essential to note that these variations are derived from different combinations of ethylene oxide, propylene oxide, and butylene oxide. In the manufacturing process, an initiator, typically alcohol, is used to graft onto these oxides, creating homo polymers. However, copolymers can also be produced, such as random copolymers where ethylene oxide and propylene oxide are combined in the mixture.
Not only can random copolymers be created, but block structures can also be formed, with a block of ethylene oxide or propylene oxide followed by a second block of the same or a different oxide. These random copolymers, block structures, and reverse block structures all exhibit different physical and tribological properties.
Furthermore, the alcohol used in manufacturing can have one, two, or three free hydroxyl groups, leading to even more variations in PAGs’ properties and applications. As a result, the architecture of the PAGs can be quite diverse, leading to their use in a wide range of applications.
Understanding the various types of PAGs and their unique properties can be quite challenging due to their diversity and the complexity of their chemical structures.
Compatibility Issues with PAGs
The use of polyalkylene glycols (PAGs) as lubricants in the industry has faced some challenges due to compatibility issues with other components, such as oils, paints, and seal materials. While PAGs have been utilized for over 75 years, operators still express concerns about the compatibility of these lubricants. This blog post will discuss the historical compatibility issues with PAGs and explore possible ways to overcome these obstacles.

PAGs can be used in various types of equipment, and none of the compatibility issues mentioned are insurmountable. When transitioning equipment from hydrocarbon oil to PAG, it is crucial to carefully follow flushing procedures due to potential incompatibility. In terms of paints and coatings, PAGs are compatible with epoxy and phenolic epoxy-type paints, but may pose challenges with alkid and vinyl paints.
Most elastomers are fairly compatible with PAGs. Fluorine-based elastomers, like Viton, are highly compatible, while NBR elastomers, such as Buna-N, are compatible when the nitrile content is high. However, low nitrile-containing seals may experience compatibility issues. It is essential to avoid using polyurethane seals, as the chemistry of PAGs is similar to that of polyurethanes. Despite these challenges, end users who transition to PAGs rarely revert to hydrocarbon oils due to the benefits that PAGs provide.
Explaining Compatibility of PAGs
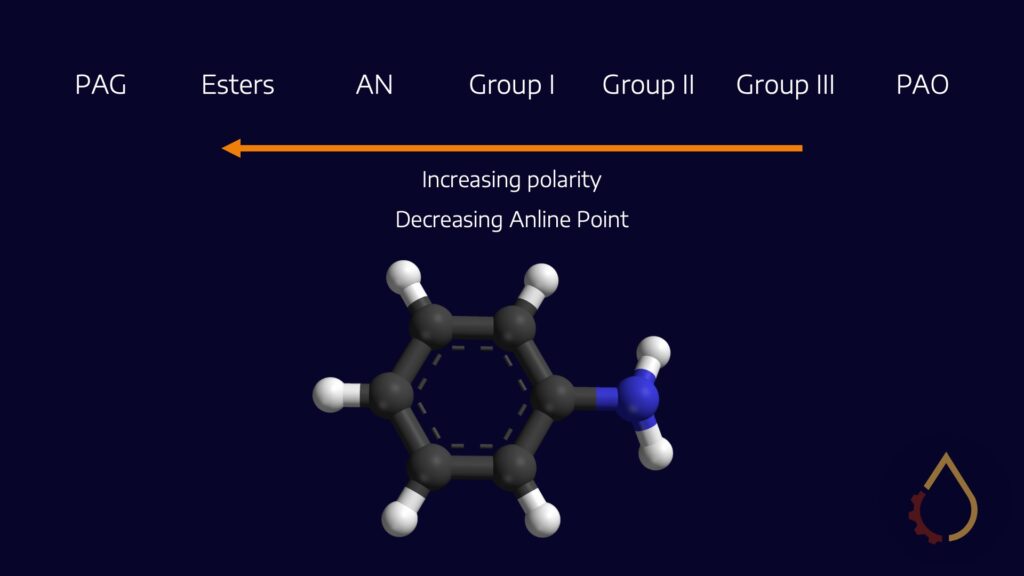
One way to explain compatibility issues with PAGs is by examining their molecular structure. Traditional oils, such as mineral and synthetic PAOs, are non-polar, and systems are designed to be compatible with non-polar fluids. In contrast, PAGs are more polar, with every third atom along the alkoxide fraction of the polymer being an oxygen atom. This molecular structure contrasts with what systems are typically designed for, causing compatibility issues with some seal materials and other components. However, the polarity of PAGs also brings significant benefits in terms of lubricant applications, including friction control, viscosity-temperature behaviour, equipment cleanliness, and more.
To mitigate compatibility issues when transitioning to PAG lubricants, operators should be diligent in selecting appropriate seal materials, paints, and coatings. Careful planning and attention to detail can help minimize potential problems while maximizing the benefits of using PAGs.
In summary, while PAG lubricants have faced compatibility challenges with other components, these issues can be managed with proper planning and care. By understanding the molecular structure of PAGs and selecting compatible materials for seals, paints, and coatings, operators can experience the advantages of PAGs without significant drawbacks. Despite initial concerns, those who transition to PAG lubricants often find the benefits outweigh the challenges and rarely return to using hydrocarbon oils.
The Future of PAGs
In recent years, the innovation in PAG (polyalkylene glycol) manufacturing seems to have slowed down. This observation can be supported by the reduced number of intellectual property publications, conference presentations, and research papers in the field. While larger PAG manufacturers may have reduced their innovation efforts, smaller, more nimble companies are looking to the future and developing new technologies in the space.
One such company, V Base Oil, is working on a new synthetic base oil technology they describe as secondary polyol esters. These innovative materials can be considered hybrid structures, combining the features of PAGs with the environmental characteristics of esters. They exhibit good biodegradability and are produced from renewable feedstocks, essentially creating a bio-based PAG in some instances. We have previously discussed bio-based esters as manufactured by the like of Biosynthetic Technologies.
As sustainability becomes increasingly important in the lubricants industry, these hybrid PAGs could represent the future of synthetic base fluids. They build on the 75 years of experience using PAGs in equipment and align with emerging trends focused on environmental responsibility.
The hybrid PAGs have a structure that combines polyol esters and PAGs. Traditional polyol esters have an ester core with long hydrocarbon chains, while the new hybrid PAGs combine polyol esters with PAG-like precursors. The result is a material with the high VI (viscosity index), hydrolytic stability, and traction behavior of PAGs, as well as the environmental benefits of esters, such as high biodegradability and renewable carbon content.
This exciting new technology could potentially revolutionize the group five synthetic base oil landscape. It addresses the challenge of replacing the sheer volume of petroleum-based lubricants with more environmentally friendly options. The longer-lasting hybrid molecules require less volume, creating a virtuous cycle in lubricant production and use.
As V Base Oil Company continues to develop and share its knowledge of these new hybrid esters, the lubricants community can look forward to advancements that marry performance with sustainability, shaping the future of the industry.
